The Changes to the European/UK VAERS Data
Subscribe to Receive Our Weekly Call To Action by Email
As of November 18, 2022 VAERS has stopped putting free text field information in for Europe/UK. This affects the narrative, the country data (previously the highest number of VAERS reports came from the UK), the history, allergies, medications and lab data.
“Disclaimer: At the request of European regulators, CDC and FDA have removed certain data fields (country codes; reported symptom case narrative free text; diagnostic laboratory data free text field; illness at time of vaccination free text field; chronic conditions free text medical history field; allergies free text field) from foreign VAERS reports which were submitted to VAERS and may not comply with European regulations. Domestic (U.S.) VAERS reports are not affected by this process.” [https://vaers.hhs.gov/data/datasets.html]
Some of our totals and charts collected data from these fields. You will notice this change throughout the site where we display all VAERS data. The domestic US data remains unaffected by this change.
The old disclaimer:

The new disclaimer as of November 18, 2022

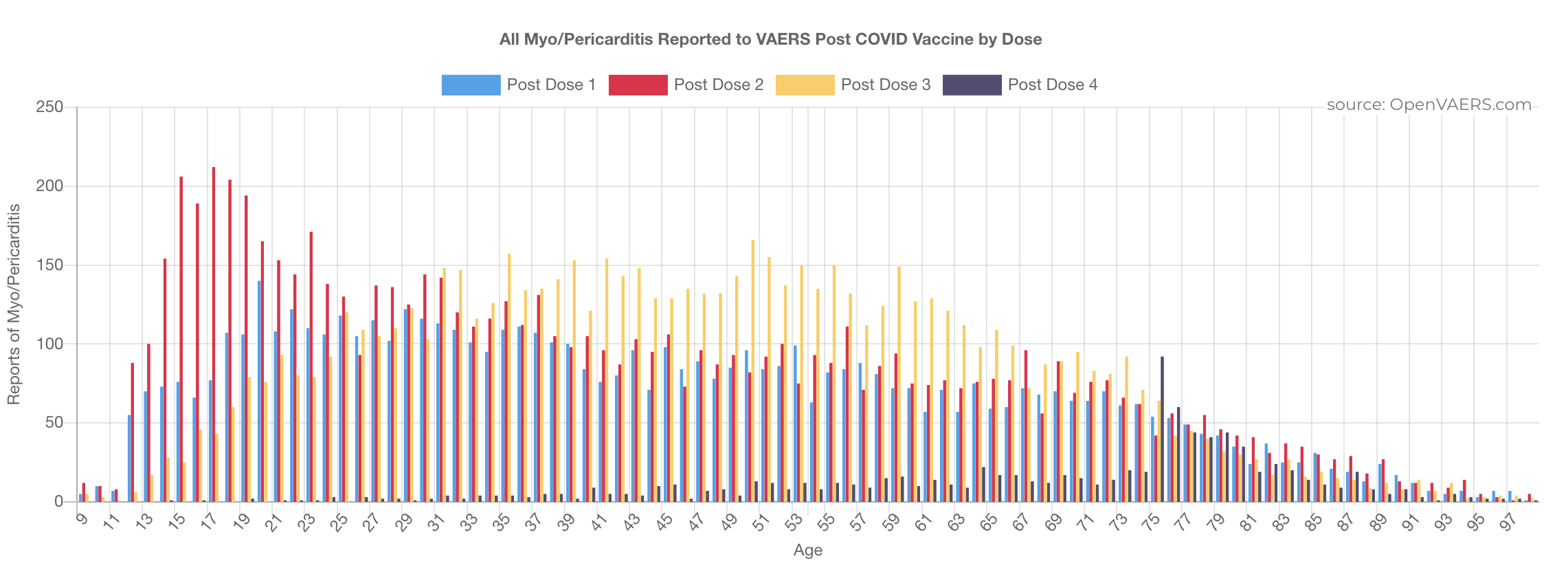
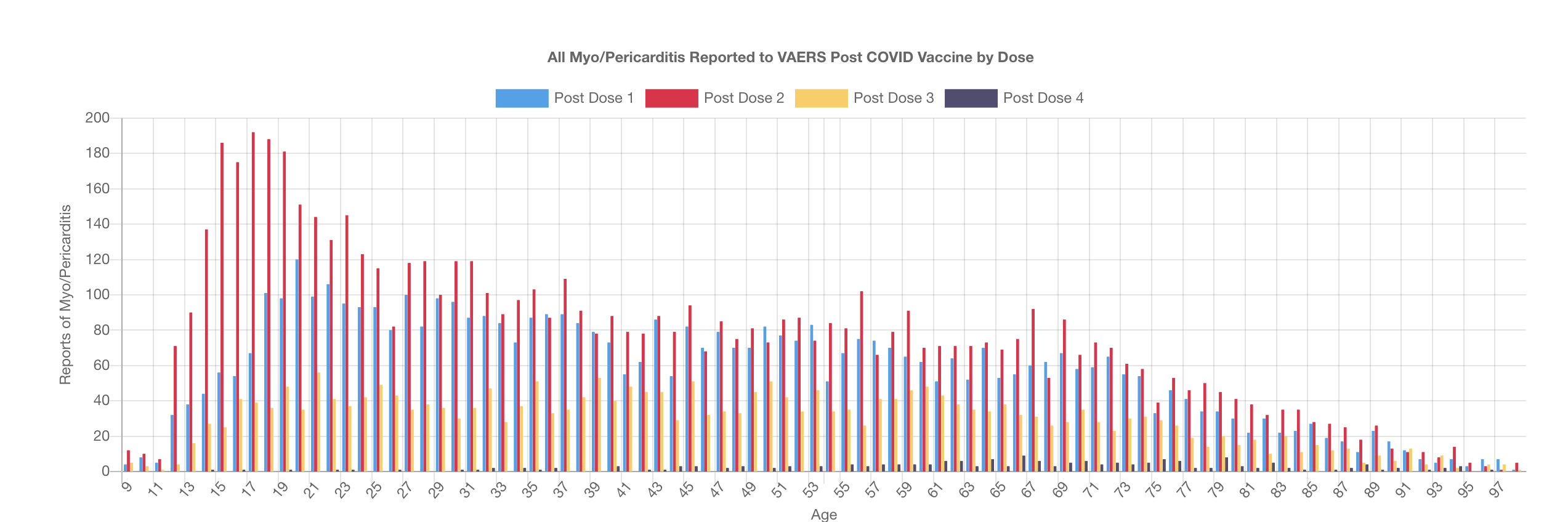
The loss of this data does not just affect our site but also researchers across the world who were able to look at patient histories and lab data and have a much more thorough understanding of the reports in question. Scientists like Dr. Jessica Rose (https://jessicar.substack.com/p/the-foreign-data-set-was-gutted-this) have lost significant signals in their data for myocarditis, cancer, miscarriage and more. Dr. Clare Craig was matching VAERS reports to MHRA reports - this will no longer be possible going forward. Aravind Mohanoor - a programmer (https://vaccinedatascience.substack.com/) was working on using the narrative to retrieve AGE data where it was missing in the AGE fields amongst some other fascinating work. And this is just the people we are aware of, there are many more.
Here is one example of what Dr. Rose's signal disappearance looks like.