VAERS, The Database, The Export, and What’s Missing
Subscribe to Receive Our Weekly Call To Action by Email
For the purposes of clarity this article will use VAERS to describe the agency that collects the injury data (which is actually not an agency but more a collaborative project co-managed by CDC and FDA lacking a head or any clearly delineated structure), the Database as the repository for that data, and the Export as the data that the public has access to.
From the beginning of our work with the VAERS Export it was clear that these were files pushed from a larger and more complete Database. For example, when we analyzed the ‘VAERS_ID’s’ we could see that over 100,000 were missing from the Export. Additionally there have been times over the years where VAERS exported incorrectly, reports appeared and disappeared, sometimes within the space of an hour. We didn’t realize the extent of the missing information until an individual (who prefers to remain anonymous) brought to our attention the process of submitting a report. The screen shots they provided unintentionally highlighted all the data being collected that we did not see in the Export. The fields can also be seen in the downloadable PDF VAERS provides for making reports.
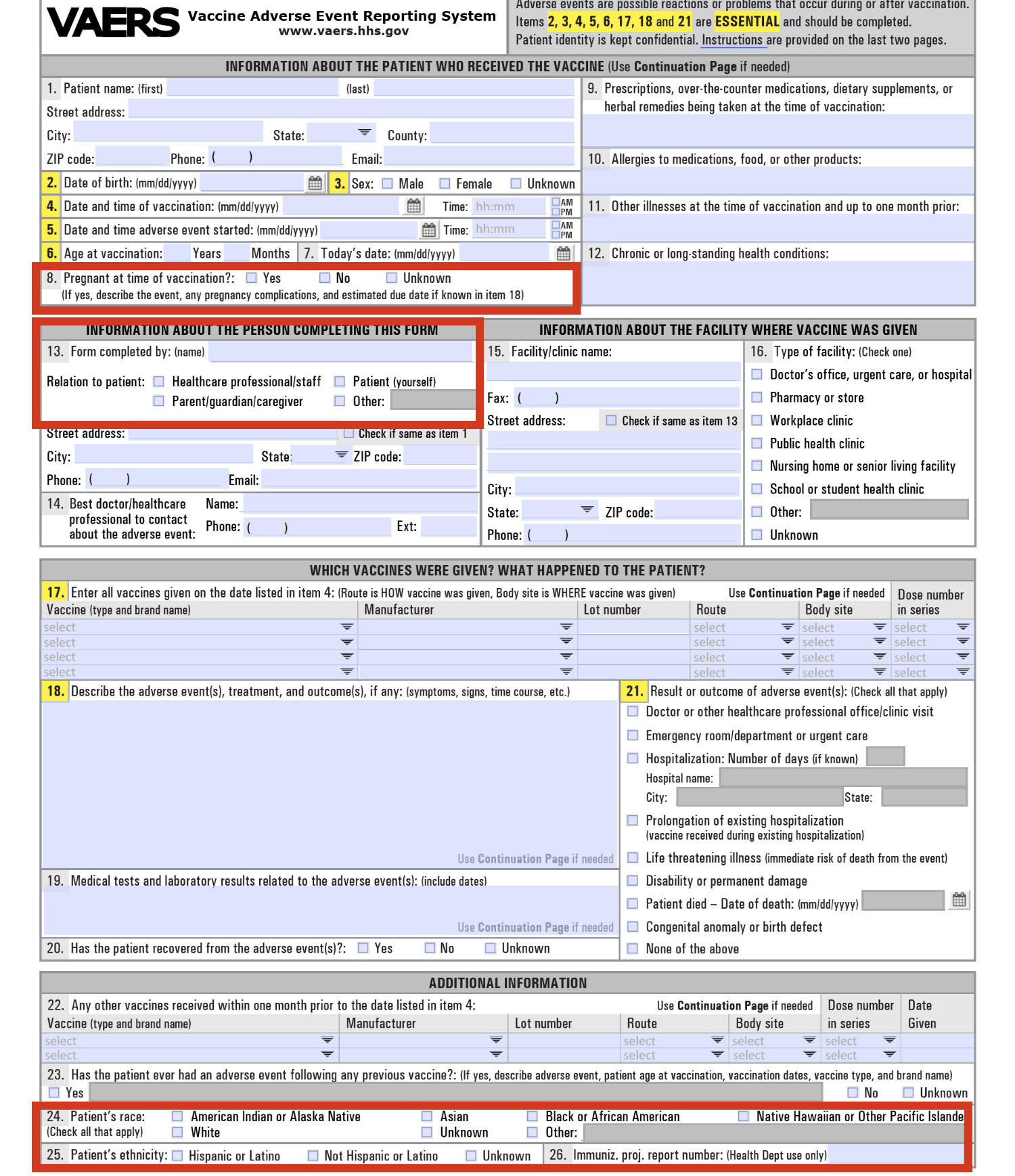 The Export does not contain the data collected by the Database on Race, Ethnicity, Pregnancy at time of vaccination, or Author. The live data on Wonder is also clearly not the full Database as it does not allow querying these fields, nor does it allow querying on other information like the ‘SPLTYPE’ which contains whether or not, for example, the report came from V-Safe (the new app set up for Covid injury tracking).
The Export does not contain the data collected by the Database on Race, Ethnicity, Pregnancy at time of vaccination, or Author. The live data on Wonder is also clearly not the full Database as it does not allow querying these fields, nor does it allow querying on other information like the ‘SPLTYPE’ which contains whether or not, for example, the report came from V-Safe (the new app set up for Covid injury tracking).
We know that VAERS is collecting the data. We know from the work of the CDC that they have access to and are viewing the data because they sometimes include race data in their reports — proof the data exists in a larger Database.[1, 2]
Finally, the article by Oster et al. [1] and the slide deck by John Su [2] give us a hint as to why the Race based data is being hidden. In both articles the reports of injuries to Black Americans and Asians is roughly equivalent. However, we learn from the CDC MMWR [3] that the rates of uptake of the COVID vaccine are very different. From Table 1 of the report, Blacks have 12.7% lower vaccination rates than whites and Asians have 10.6% higher vaccination rates. If that is the case then the overall rate of vaccine injury in the Black population is much higher than in the White or Asian population. This would be consistent with a large body of research that shows a different immune response by race (and sex) for some vaccines.[4-7] OpenVAERS calls upon the CDC to release all VAERS data to the public so that independent scholars can conduct their own research on race and sex effects from Covid-19 and other shots.
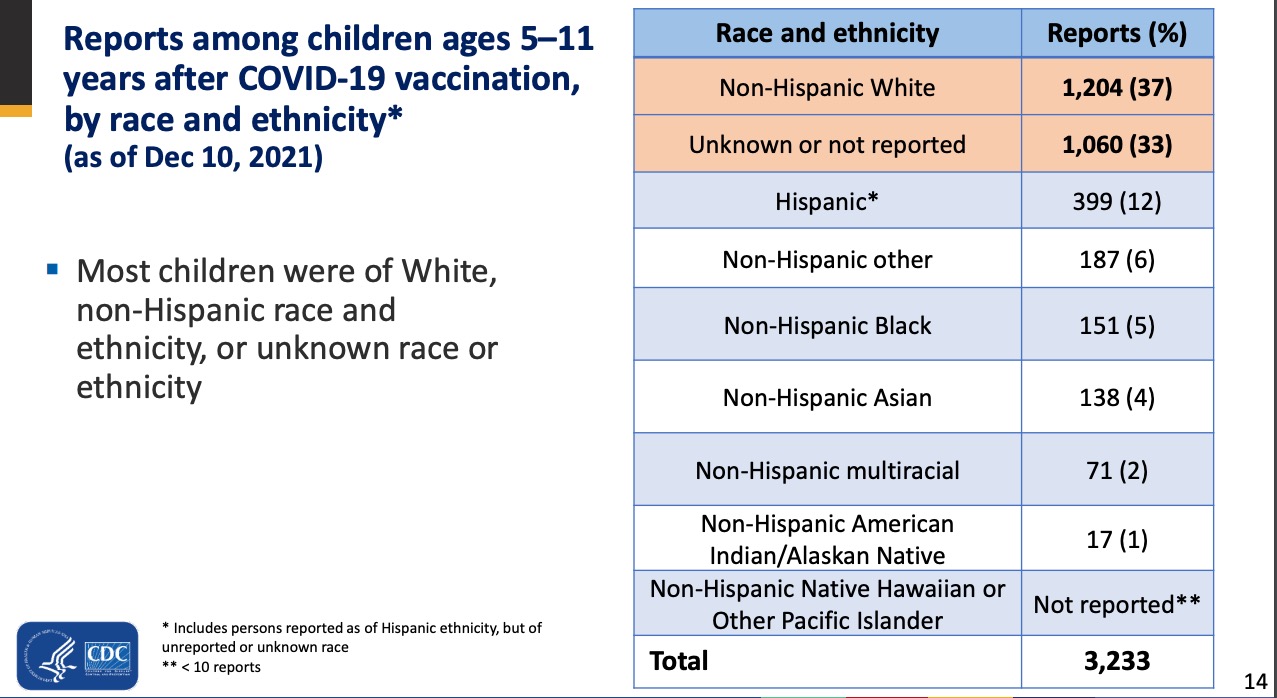
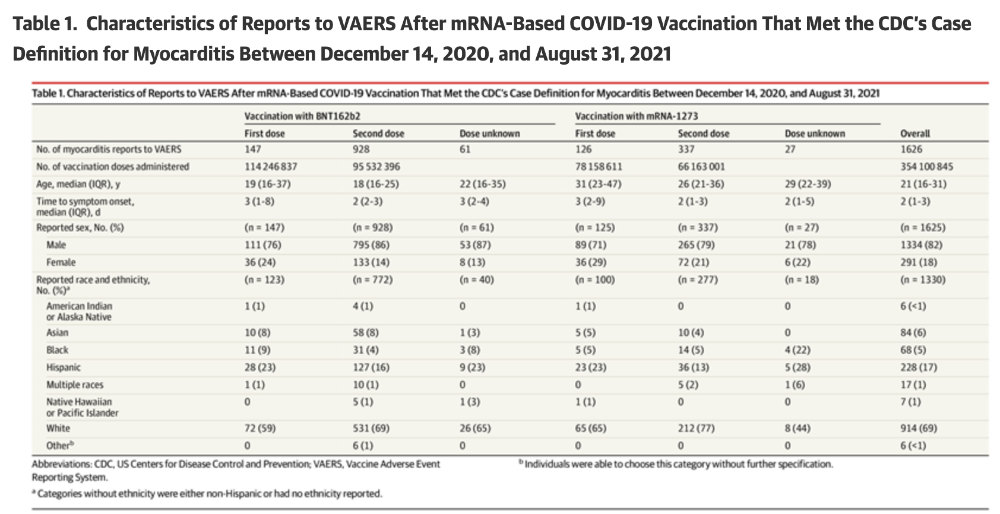
- https://jamanetwork.com/journals/jama/article-abstract/2788346
- https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-12-16/05-COVID-Su-508.pdf
- https://www.cdc.gov/mmwr/volumes/71/wr/mm7123a2.htm#T1_down
- http://newsnetwork.mayoclinic.org/discussion/mayo-clinic-discovers-african-americans-respond-better-to-rubella-vaccine/
- https://doi.org/10.1016/j.vaccine.2014.01.090
- https://www.ebiomedicine.com/article/S2352-3964(17)30046-4/abstract
- https://doi.org/10.1080/15287394.2010.519317